HOW OLD IS IT? |
|---|
HOW OLD IS IT? |
|---|
The absolute age of a wide variety of rocks, fossils, and human artifacts can be determined by various laboratory procedures. Deciding which test to perform depends on the composition and believed approximate age of a particular specimen.
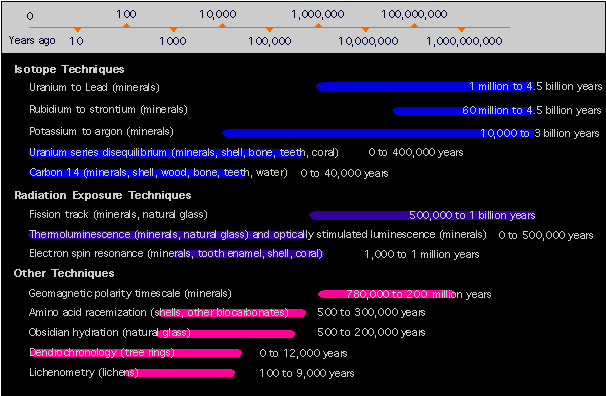
For example Carbon 14 is often the method of choice for scientists dating organic artifacts like wood, charcoal, bone, and teeth that are less than about 40,000 years. Carbon 14 as an isotope exists in Earth's atmosphere at more or less constant concentrations relative to other carbon isotopes (there are exceptions, which published tables compensate for). When living tissue dies the Carbon 14 begins to slowly decay. Carbon 14 has a half-life of about 5,370 years, meaning that every 5,370 years about 50% of it decays to it's daughter product, the Carbon 12 isotope. After another 5,370 years only 25% of it remains, and so on. After 40,000 years there isn't enough of it left to accurately measure, so to date anything much older requires use of one or more of the other age-determination methods.
The Carbon 14 test would be inappropriate for something like, say, granite because what we already know about granite is that it is too old and of the wrong composition for that test. But the granite may very well contain traces of the elements Uranium, Rubidium, or Potassium, and a test using one or more of those isotope procedures may well be appropriate and yield good results.
Some of the techniques for determining absolute age are listed in the graphic below, adapted from a September, 2001 National Geographic Magazine chart and article.
NOTE: The timeline scale is a logarithmic representation
Credits:
National Geographic Magazine, September, 2001
Original Source:
W. Jack Rink, McMaster University, Ontario.
|| Geologic Time Scale || Table of Contents ||